A watch battery, button cell, or coin battery is a small single cell battery shaped as a squat cylinder typically 5 to 25 mm (0.197 to 0.984 in) in diameter and 1 to 6 mm (0.039 to 0.236 in) high — resembling a button.
The button cell, also known as the coin cell, enabled compact design in portable devices of the 1980s. Higher voltages were achieved by stacking the cells into a tube. Cordless telephones, medical devices, and security wands at airports used these batteries.
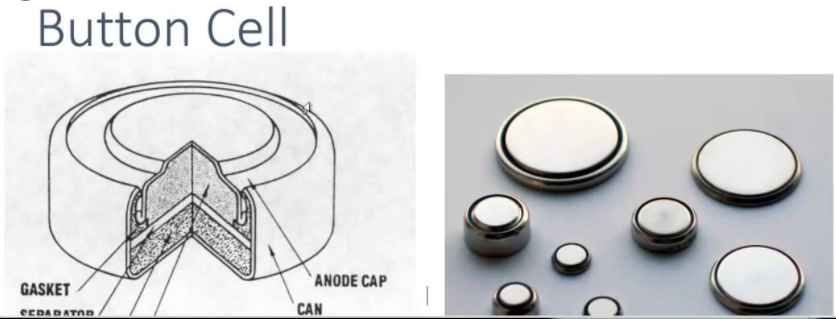
Most button cells in use today are non-rechargeable and are found in medical implants, watches, hearing aids, car keys, and memory backup
Cells of different chemical composition made in the same size are mechanically interchangeable. However, the composition can affect the service life and voltage stability. Using the wrong cell may lead to short life or improper operation.
Sometimes different cells of the same type and size and specified capacity in milliampere hour (mAh) are optimized for different loads by using different electrolytes, so that one may have longer service life than the other if supplying a relatively high current.
Button cells are very dangerous for small children. Button cells that are swallowed can cause severe internal burns and significant injury or death.

Caution:
Keep button cells to out of reach of children. Swallowing a cell can cause serious health problems
Button cells are single cells, usually disposable primary cells. Common anode materials are zinc or lithium. Common cathode materials are manganese dioxide, silver oxide, carbon monofluoride, cupric oxide or oxygen from the air. Mercuric oxide button cells were formerly common, but are no longer available due to the toxicity and environmental effects of mercury.

